The “potential stocks†of the Chinese pharmaceutical market – drugs with obvious clinical value
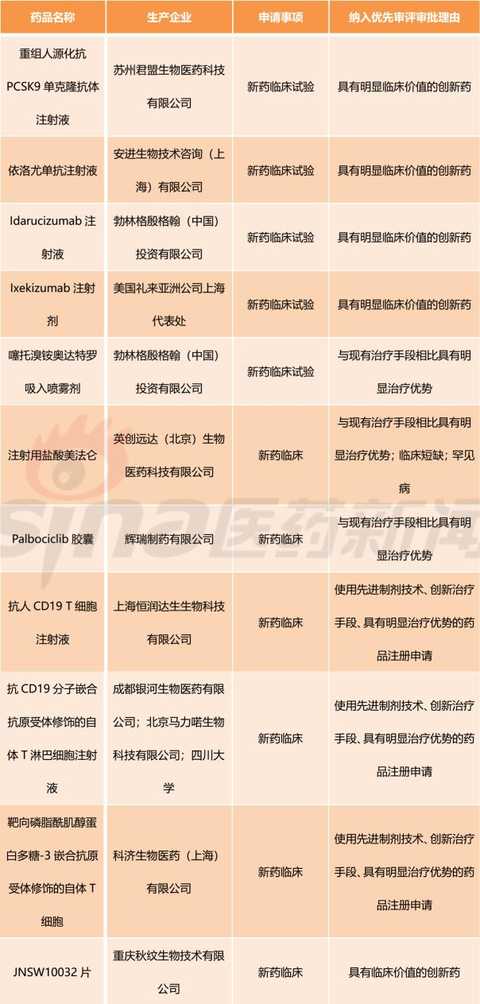
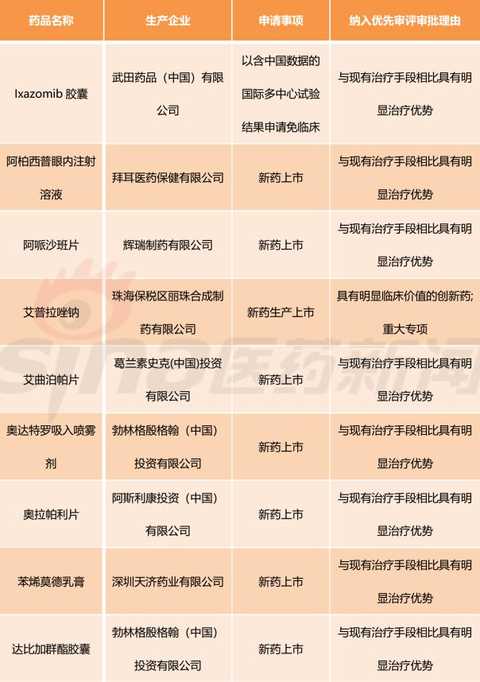
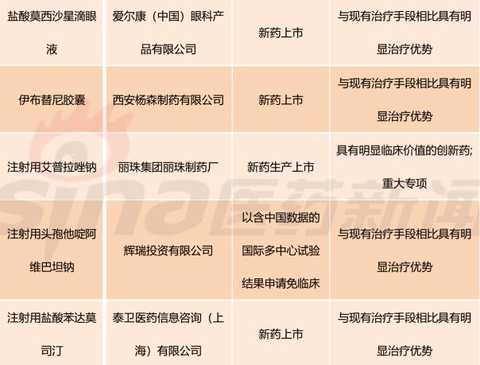
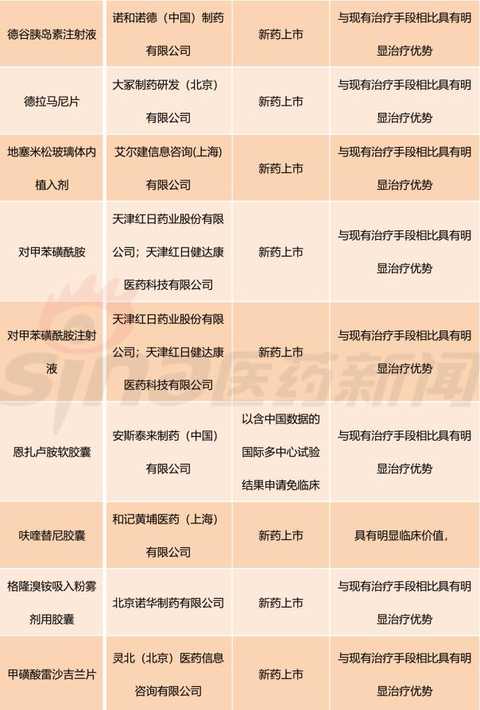
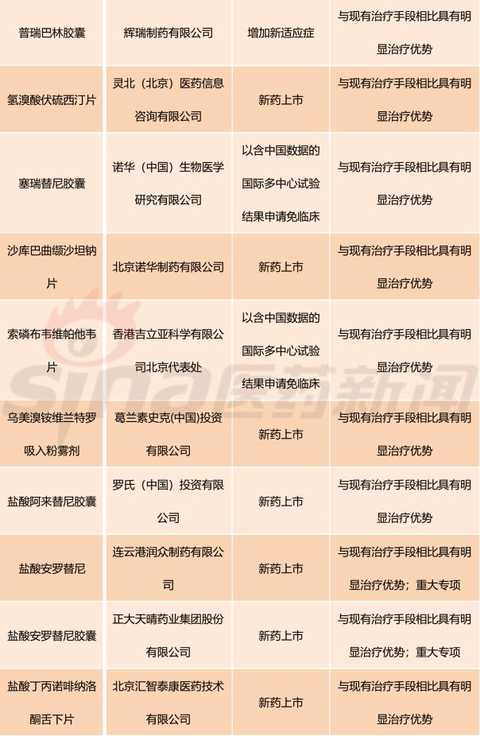
[Hui Cong Pharmaceutical Industry Network] The basis of drug listing is clinical value. In recent years, the country has paid more and more attention to the clinical value of new drug market. The State Council's Opinions on Reforming the Examination and Approval System for Pharmaceutical Medical Devices in 2015 (Guo Fa [2015] No. 44) clearly stated that it encourages clinical value-oriented drug innovation. The 2017 edition of the Drug Registration Management Measures (Revised Draft) also clearly sets out the drug review requirements based on the clinical value of the drug. Therefore, the development and review of innovative drugs should be guided by clinical value, focusing on the novelty and originality of the material foundation, and should pay more attention to the evaluation of clinical value. There is currently no definitively defined definition of the clinical value of a drug. Most understand from clinical needs that the clinical value of a drug is the extent to which the drug meets medical and clinical needs. In my opinion, the main purpose of new drug research and development is to meet clinical needs. Compared with existing medical measures, new drugs have a significant clinical value by filling existing clinical treatment gaps or having significant advantages over existing treatment methods. Although there are no precise definitions for drugs with significant therapeutic advantages, they generally fit the following aspects: It has the advantage of major clinical efficacy beyond the existing treatment methods, that is, the curative effect is high, and the adverse reactions are within an acceptable range. The drugs are often used as first-line treatment drugs or preferred drugs in the clinic. Some drugs, although the efficacy is similar to the existing standard treatment methods, but compared with the original treatment drugs, the adverse effects of new drugs are significantly reduced. Some drugs are used to treat patients who are allergic or non-responsive to existing treatments, ie to fill the current treatment gap. For drugs with significant clinical value, the CFDA has established a priority review and approval “road color channelâ€. On December 28, 2017, the CFDA official website issued the “Opinions on Encouraging Drug Innovation to Implement Priority Review and Approvalâ€. The scope of priority review and approval is shown in Table 1. From Table 1, we can clearly see that having obvious clinical value is a core element of the priority approval review. The author has compiled a list of priority review and approval issued by the CFDA in the past two years, and selected drugs with obvious clinical value for reference. The author has compiled a total of 13 batches of priority review and approval lists issued by the CFDA from October 28, 2016 to March 28, 2018. A total of 301 acceptance numbers, 98 of which were included in the priority review list due to their obvious clinical value. , accounting for about 1/3. Based on the generic name of the drug, there are 11 new drug clinical trial applications, and 40 new drugs are available for marketing. The new drug clinical trial application is shown in Table 2. It can be seen that there are 7 biological drugs and 4 chemical drugs. The biopharmaceutical contains monoclonal antibody and more advanced T cell therapy as well as CAR-T. For example, recombinant humanized anti-PCSK9 monoclonal antibody injection developed by Suzhou Junmeng Biomedical Technology Co., Ltd.; targeted phosphatidylinositol-3 polysaccharide antigen receptor modification developed by Keji Biomedical (Shanghai) Co., Ltd. Autologous T cells. From the point of view of production enterprises, we can see international multinational companies such as Anjin, Boehringer Ingelheim, and Lilly, as well as many innovative and innovative pharmaceutical companies such as Yingchuang Yuanda and Shanghai Hengrunda. This shows that China has made great progress in the field of biomedical innovation. Table 2: Application for clinical trials of innovative drugs with priority clinical review and approval (2016/10/28-2018/03/28) The application for new drug listing is shown in Table 3. From the perspective of enterprise distribution, major pharmaceutical giants have basically submitted applications for approval of drugs. The top three are Boehringer Ingelheim, Novartis, AstraZeneca and Johnson & Johnson. The news that these multinational pharmaceutical companies are more promising is that their products were included in the scope of medical insurance in some areas shortly after they were approved. In December 2017, Novartis's rocotinib and pizopanib, AstraZeneca's Oxytinib, Johnson's Ibbutinib, BMS's Dalatinvir/Ashuvire, Roche's Vermofinil It was included in the scope of major illness insurance in Zhejiang Province. Novartis's Shakuba / valsartan, BMS's Dalatavivir / Ashuravir were also included in the province's Category B medical insurance catalogue in January 2018. At the same time, under the encouragement of a series of new drug research and development policies, China's innovative drug research and development capabilities have made great progress. Here I choose a few drugs to explain a little. Developed by Hutchison Whampoa Pharmaceuticals, furazolinib is a targeted drug for the treatment of advanced colorectal cancer. Colorectal cancer is a high-grade malignant tumor in China and the world. The treatment of advanced colorectal cancer is mainly chemotherapy, and combined chemotherapy is used. Patients with colorectal cancer who failed first-line treatment were mainly treated with second-line chemotherapy, but the follow-up treatment plan for patients after the failure of current second-line standard treatment is still lacking. Furazolinib is expected to be a new choice for patients with advanced colorectal cancer. Pyrrolidine maleate developed by Hengrui is an oral anti-cancer targeted drug. The current indications for the application are Her-2 positive metastatic/advanced breast cancer and Her-2 mutant advanced non-small cell lung. Adenocarcinoma and Her-2 positive for advanced gastric cancer. Among them, breast cancer indications are the main factors. An anti-tumor 1.1 new drug, erlotinib hydrochloride, independently developed by Zhengda Tianqing Pharmaceutical Group, is a novel small molecule multi-target tyrosine kinase inhibitor that can effectively inhibit VEGFR, PDGFR, FGFR, c-Kit, etc. Kinase, which has anti-tumor angiogenesis and inhibits tumor growth. The drug was approved by the US FDA in 2015 for the approval of orphan drugs for ovarian cancer. The indication for this report is non-small cell lung cancer (NSCLC). At present, a variety of cancer clinical trials are underway, including non-small cell lung cancer, soft tissue sarcoma, gastric cancer, colorectal cancer, medullary thyroid carcinoma, differentiated thyroid cancer, and esophageal squamous cell carcinoma. The iprazol sodium for injection developed by Livzon Group belongs to the exclusive variety and independently developed innovative drugs. The indication is peptic ulcer bleeding, which has the advantages of long acid suppression time, small individual differences and less drug interaction. Take the medicine once. Currently available domestic proton pump inhibitors include omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole, and iprazol. According to IMS statistics, domestic azole injections The 2016 annual sales were approximately RMB 16.168 billion. Compaq Xipu Ophthalmic Injection, developed by Chengdu Kanghong Biotechnology Co., Ltd., is a new class I therapeutic drug for biological products. It is a fusion protein of VEGF receptor and human immunoglobulin Fc segment gene. Vascular endothelial growth factor (VEGF) competitively inhibits the binding of VEGF to receptors and prevents the activation of VEGF family receptors, thereby inhibiting endothelial cell proliferation and angiogenesis, and achieving the goal of treating wet age-related macular degeneration. Compaq Xipu Ophthalmic Injection is the first self-developed drug for the treatment of this disease in China, and has positive significance for solving the accessibility of clinical drugs in China. Of course, there are still many applications for new drug listings, and I will not repeat them here. These drugs, which have been clinically valued and have been included in the priority review and review by CFDA, are the "essence" of new drugs listed in China in the past two years, and are also an excellent "window" for observing the development of China's pharmaceutical innovation. In short, their emergence will continue to fill the gap in China's medical needs and improve the level of medical security. Table 3: Application for the listing of innovative drugs with priority clinical review and approval (2016/10/28-2018/03/28) Editor in charge: Yang Manman pvc leather for bag, pu leather for bag, artifical leather for bag, synthetic leather for bag, bag leather, Faux Leather Suitcase,Leather For Wallet,Faux Leather Bag,Artificial Leather For Bag Vigor Plus Co., Ltd , https://www.vigorplusx.com